Iron as a key redox species in microbiological processes
Theme 2
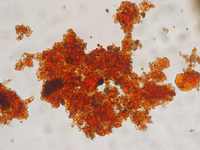 A connection between microbial activity and redox transformations of iron has been postulated by C.G. Ehrenberg already more than 170 years ago based on his microscopic studies of bog iron ores. However, only in the last decades the relevance of microbial transformations for the cycling of iron in nature has become more and more evident. Thanks to the development of new cultivation methods it became possible to isolate iron oxidizing or reducing bacteria and to study their metabolic capabilities. Enormous progress in molecular biology provided new insight into the complex microbial ecology of microorganisms involved in iron cycling.
A connection between microbial activity and redox transformations of iron has been postulated by C.G. Ehrenberg already more than 170 years ago based on his microscopic studies of bog iron ores. However, only in the last decades the relevance of microbial transformations for the cycling of iron in nature has become more and more evident. Thanks to the development of new cultivation methods it became possible to isolate iron oxidizing or reducing bacteria and to study their metabolic capabilities. Enormous progress in molecular biology provided new insight into the complex microbial ecology of microorganisms involved in iron cycling.
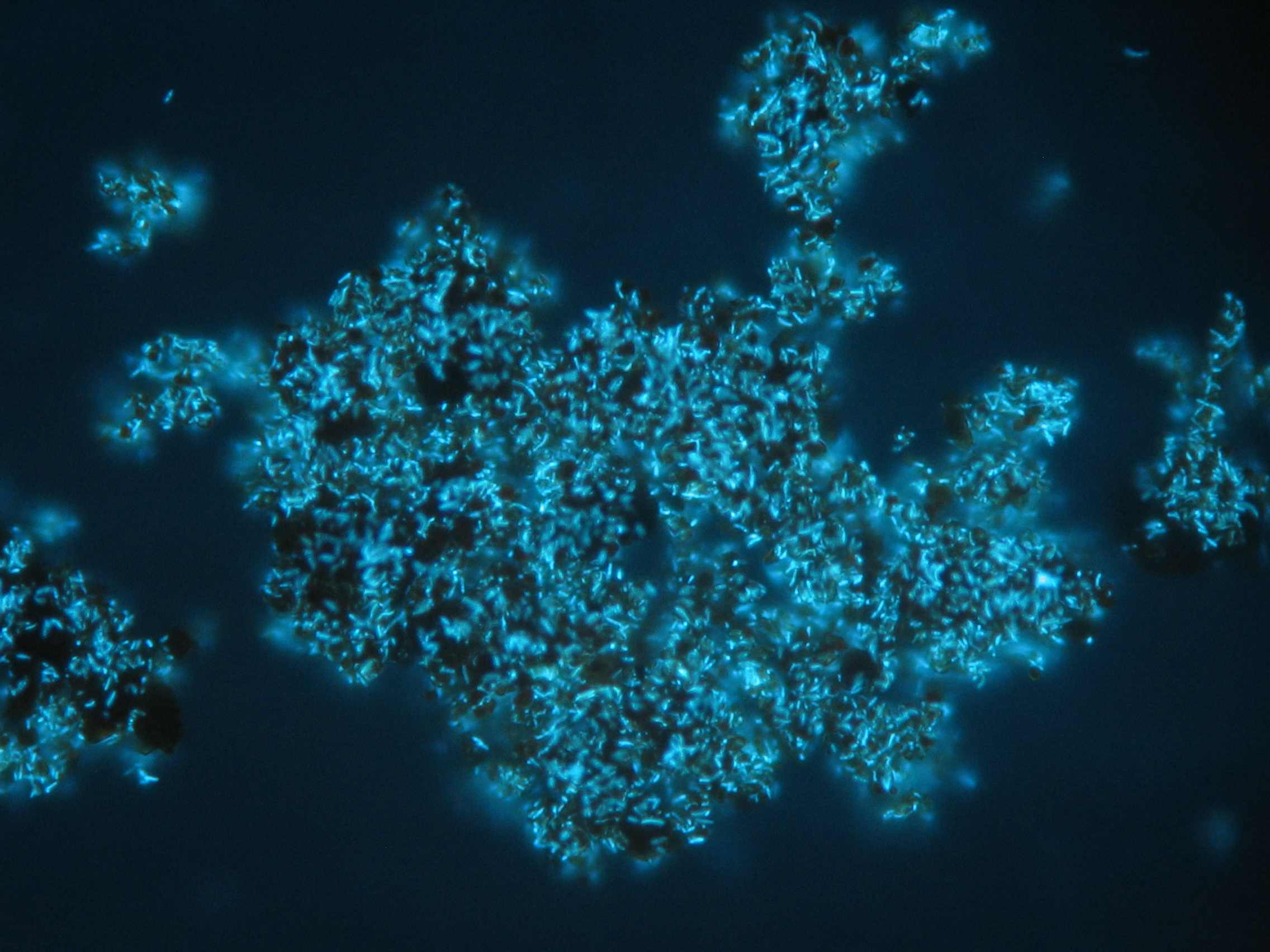 However, this development is still ongoing and many questions regarding the microbial role in the iron cycle in natural environments still remain open. For example, the quantitative significance of microbial mediated iron transformations is still unclear because microbial processes often compete with abiotic reactions and their individual contributions are difficult to separate. It appears that many species use naturally occurring organic redox shuttles, e.g., humic acids for transfer of electrons to the iron oxide surface, the kinetics of which are not well understood nor are the thermodynamics. It appears that both the redox potential of the bulk mineral and also the particle size regulate the extent of such mechanisms. Moreover, passivation of the surface by dissolved organic matter or inorganic species may interfere with electron shuttling processes. Also our understanding of the diversity of microorganisms contributing to iron transformations in natural environments is still in its infancy and linking microbial community structure with its functionality yet needs to be resolved.
However, this development is still ongoing and many questions regarding the microbial role in the iron cycle in natural environments still remain open. For example, the quantitative significance of microbial mediated iron transformations is still unclear because microbial processes often compete with abiotic reactions and their individual contributions are difficult to separate. It appears that many species use naturally occurring organic redox shuttles, e.g., humic acids for transfer of electrons to the iron oxide surface, the kinetics of which are not well understood nor are the thermodynamics. It appears that both the redox potential of the bulk mineral and also the particle size regulate the extent of such mechanisms. Moreover, passivation of the surface by dissolved organic matter or inorganic species may interfere with electron shuttling processes. Also our understanding of the diversity of microorganisms contributing to iron transformations in natural environments is still in its infancy and linking microbial community structure with its functionality yet needs to be resolved.
Identification of biogeochemical processes in sedimentary or soil samples can be performed by measuring stable isotopes ratios of Fe. Some of the largest fractionations in the isotopic composition of iron occur between oxidized and reduced forms. Because biochemistry involves changes in redox state, this fractionation process has been a major motivation for developing this isotopic system as a means for tracing biogeochemical phenomena. In environments that contain iron in both oxidation states, the oxidized form is generally enriched in the heavy isotopes on the order of several per mil (‰) at room temperature.



