Environmental biogeochemistry of iron
Theme 3
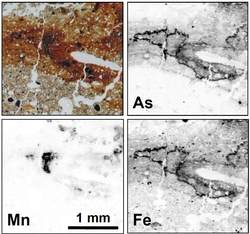 Iron oxide and oxyhydroxide minerals in the environment are important sorbents for many trace metals (e.g., Cu, Pb, Zn) and metalloids (As, Sb). They strongly influence trace element mobility, bioavailability, and cycling in terrestrial and aquatic systems. Due to its extremely large surface area, poorly crystalline Fe(III) minerals are of particular importance. For example, the schwertmannite, a ferric hydroxyl sulfate mineral, which is commonly formed in environments affected by acid mine drainage, can retain significant fractions of the metals and metalloids released by sulphide oxidation in these systems.
Iron oxide and oxyhydroxide minerals in the environment are important sorbents for many trace metals (e.g., Cu, Pb, Zn) and metalloids (As, Sb). They strongly influence trace element mobility, bioavailability, and cycling in terrestrial and aquatic systems. Due to its extremely large surface area, poorly crystalline Fe(III) minerals are of particular importance. For example, the schwertmannite, a ferric hydroxyl sulfate mineral, which is commonly formed in environments affected by acid mine drainage, can retain significant fractions of the metals and metalloids released by sulphide oxidation in these systems.The figure illustrates the close association of arsenic with iron in a contaminated river floodplain soil. When the soil is oxic, arsenic is present mainly as As(V) sorbed to poorly crystalline Fe(III) hydroxides. Prolonged flooding, however, induces anoxic conditions favouring microbial reduction and dissolution of Fe(III) hydroxides, reduction of As(V) to As(III), and release of Fe(II) and As(III) into pore water. Understanding the processes governing the kinetics of microbial iron and arsenic reduction and release in soils and sediments are currently active research fields in environmental biogeochemistry.



