Binding of Antimony to Natural Organic Matter in a Finnish Mine-Water Influenced Peatland
2 Water Resources and Environmental Engineering Research Unit, University of Oulu, Oulu, Finland
3 Geological Sciences, Stanford University, Stanford, CA, USA
4 SSRL, Stanford University, Stanford, CA, USA
5 The Rossendorf Beamline at ESRF, Grenoble, France
6 Earth System Sciences, Stanford University, Stanford, CA, USA
O 1.3 in The Skin of the Earth and below: Soil and Water
11.10.2018, 09:40-09:55, H36, NW III
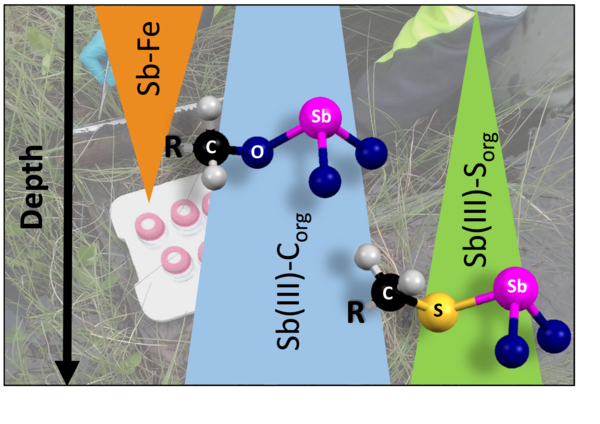
Antimony (Sb) is a toxic element typically of low natural abundance, but human activities have led to highly elevated concentrations in many soils and sediments. Recently, natural organic matter (NOM) has been discussed as an effective sink for arsenic [1], and first spectroscopic studies [2,3] indicated that sulfhydryl moieties of NOM also play an important role in controlling Sb binding in wetland sediments. However, Sb speciation in NOM-rich wetlands has not yet been studied comprehensively and direct spectroscopic evidence for this sequestration mechanism is still lacking. In order to investigate the role of NOM in Sb sequestration, we used bulk Sb K-edge X-ray absorption fine structure (EXAFS) spectroscopy to interrogate Sb in a northern Finland peatland which is influenced by an adjacent gold mine. Sampled peat cores were kept under an argon atmosphere at 4 ºC in the dark until freeze-drying to prevent Sb speciation changes. The peat contained up to 52 % carbon and 265 mg/kg Sb (dry weight basis). Sulfur and iron contents ranged between 4 to 8 and 2 to 10 g/kg, respectively. Aqueous Sb concentrations decreased with lateral distance from the inflow from 190 μg/L in surface waters to 8 μg/L in 80 cm depth. Based on linear combination fittings of EXAFS spectra, we found Sb to be mainly coordinated to NOM moieties in all peat samples. In 10-40 cm depth, Sb was sorbed up to 29% to iron minerals and with increasing depth, up to 36% of trivalent Sb was complexed tri-fold to sulfhydryl moieties of NOM. Together with complexation of up to 66% to alcoholic groups of NOM, Sb was up to 100% complexed to NOM in the deeper peat-layers. Our results show that sorption of Sb to particulate NOM can act as an important sequestration mechanism under sulfate reducing conditions and therefore strongly influences Sb mobility in the environment.
[1] Langner et al. (2012) Nat. Geosci. 5, 66-73. [2] Benett et al. (2017) Environ. Chem. 2017, 14, 345–349. [3] Arsic et al. (2018) Environ. Sci. Technol. 52, 1118-1127.
Keywords: Antimony Sequestration Natural Organic Matter X-ray absorption spectroscopy
Export as iCal: