We focus on the diversity, structure and function of natural microbiomes in soil and in freshwater habitats. We work in a wide range of natural ecosystems including agricultural soils, streams and lakes, groundwater and even in some exotic places such as caves and mines. Some of our projects also address engineered water systems such as waste water treatment and drinking water production. The investigated microbial community functions directly relate to water quality, the turnover of pollutants and nutrients, and to microbe-plant interactions in the rhizosphere.
Much of the research in our group is based on the so-called “key population” concept, advocating that only defined components of the complex microbiota found in environmental systems are actually relevant for specific processes. Absence or loss of such key populations may cause a loss of function. At the same time, the targeted identification and characterisation of such key populations is pivotal to understand the controls of environmental processes, such as pollutant degradation in groundwater or nutrient cycling in the rhizosphere.
Microbial turnover of pollutants in ground- and surface waters
Understanding the ecology and functional capacities of pollutant-degrading microbial populations in anthropogenically impacted natural ecosystems is a central aim of our group. Using tools of molecular microbial ecology, we investigate which microbial key-players are relevant for pollutant degradation in situ, and which environmental and ecological factors are controlling the activity and diversity of these populations. We currently focus on the following processes:
Microbial nitrate elimination
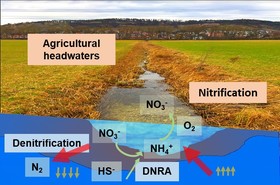 Increasing anthropogenic nitrogen loading in agricultural catchments is a serious threat to our water resources. While the ecophysiologies of reductive or oxidative nitrogen cycling are well understood at the microbiological level, the controls of these processes and populations are still poorly understood at the watershed level. Nitrate loading is a major problem especially in agricultural headwaters. In our research, we investigate how the interplay of bidirectional water exchange fluxes between groundwater and the stream and nitrogen-cycling microbial populations established in the streambed influence nitrate elimination via denitrification, and also potential secondary nitrate loading via nitrification.
Increasing anthropogenic nitrogen loading in agricultural catchments is a serious threat to our water resources. While the ecophysiologies of reductive or oxidative nitrogen cycling are well understood at the microbiological level, the controls of these processes and populations are still poorly understood at the watershed level. Nitrate loading is a major problem especially in agricultural headwaters. In our research, we investigate how the interplay of bidirectional water exchange fluxes between groundwater and the stream and nitrogen-cycling microbial populations established in the streambed influence nitrate elimination via denitrification, and also potential secondary nitrate loading via nitrification.
Microbial hydrocarbon degradation
We work on the microbial degradation of petroleum hydrocarbons, using toluene as a model system. We have pioneered the development of a widely applicable catabolic marker gene assay targeting a fragment of the benzylsuccinate synthase alpha-subunit (bssA) gene, the key-enzyme of anaerobic toluene degradation.
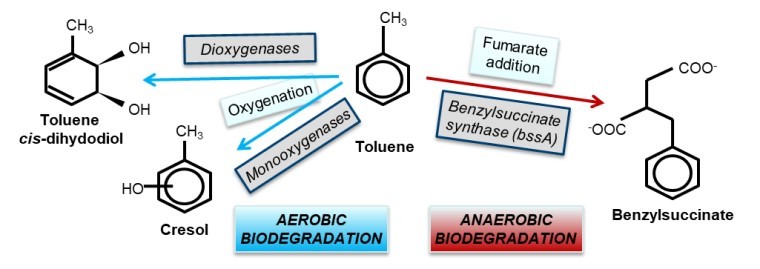
We seek to understand how the diversity in a given catabolic gene pool relates to the ecological niches potentially filled by local degraders. Moreover, we investigate the microbial biodegradation strategies in the “gray” area between aerobic and anaerobic biodegradation, i.e. in scenarios where oxygen and anaerobic electron acceptors are of limited simultaneous or transient availability. These insights are important for the development of improved monitoring and bioremediation strategies for contaminated sites.
Microbial activities and interactions in the rhizosphere
Rhizosphere microbial communities are well known to affect the health and growth of plants in many ways. In our research, we investigate how the composition of rhizosphere microbiota (incl. bacteria, archaea and microeukaryotes) and the activities of specific populations within relates to soil fertility, plant stress tolerance, and the allocation of plant-derived carbon in soil. Our research currently involves two projects in this field:
Rhizosphere microbiome trait plasticity
Within the BMBF-funded “RhizoTraits” project, we investigate how the microbiome of modern, yield optimized varieties of maize differs compared to that of old landraces. We hypothesize that plant traits fostering the formation of a plant-beneficial microbial community may have been compromised in the breeding of modern varieties. Focusing on drought stress tolerance as one important parameter, we systematically compare a large selection of different maize varieties under greenhouse conditions and at distinct field sites. Together with our project partners, we aim to develop a holistic understanding of the mechanisms and functions within the plant-microbe system under water limitation. This is a basis for the consideration of rhizosphere microbiome traits in the development of future crop varieties and agricultural practices.
https://www.bayceer.uni-bayreuth.de/rhizotraits/
Microplastic pollution in the Rhizosphere
 The input of plastics, especially microplastics (MP) into agricultural soils is of increasing global concern, but the effects on soil functioning and on plant growth are not yet well understood. MP can disrupt natural processes and functions in soil, especially in the rhizosphere, and lead to soil aggregate destabilization, reduce soil humus, nutrients and moisture, and alter the activities of rhizosphere soil microbiota. However, the effects of MP on these important soil properties, soil microorganisms and plant health have so far been investigated with partially contradicting conclusions. In our project within the DFG-funded CRC “Microplastics”, we are working with maize and strawberries as agricultural model plants. We aim to generate a more solid understanding of the impairment of soil functions by MP, of changes in rhizosphere microbiota and potentially stimulated degradation of plastics in the rhizosphere, as well as of a potential long-term degradation of soil quality by microplastic pollution.
The input of plastics, especially microplastics (MP) into agricultural soils is of increasing global concern, but the effects on soil functioning and on plant growth are not yet well understood. MP can disrupt natural processes and functions in soil, especially in the rhizosphere, and lead to soil aggregate destabilization, reduce soil humus, nutrients and moisture, and alter the activities of rhizosphere soil microbiota. However, the effects of MP on these important soil properties, soil microorganisms and plant health have so far been investigated with partially contradicting conclusions. In our project within the DFG-funded CRC “Microplastics”, we are working with maize and strawberries as agricultural model plants. We aim to generate a more solid understanding of the impairment of soil functions by MP, of changes in rhizosphere microbiota and potentially stimulated degradation of plastics in the rhizosphere, as well as of a potential long-term degradation of soil quality by microplastic pollution.https://www.sfb-mikroplastik.uni-bayreuth.de
Microbial biofilms in caves and mines
Caves and mines represent unique subsurface ecosystems largely devoid of light-driven primary production and offer a remarkable glimpse into the investigation of microbial life in the subsurface. Especially, as-yet undescribed microbial diversity and enigmatic metabolic capacities have been shown to be found in subsurface biofilms, the formation of which is often driven by chemolithoautotrophic processes.
Since several years, we are investigating the massive biofilm formation discovered in a peculiar methane-fuelled, iodine-rich former medicinal spring in Southern Germany. We aim to elucidate the drivers of this biofilm formation and unravel potential couplings between biogeochemical methane- and iodine-cycling in this very special microbe-dominated subsurface system. Moreover, we have recently started to investigate manganese-oxidizing microbial biofilms in a historic mining system, as well as peculiar microbial calcite precipitations found in a karstic cave, both in the direct vicinity of Bayreuth.

Labelling-assisted targeted Environmental ‘Omics’
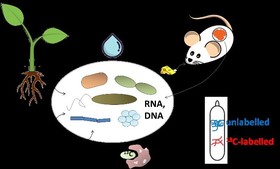 To a large part, our research is driven by the development and application of advanced molecular ‘omics’ technologies in combination with targeted labelling, where our group hosts a distinctive and cutting-edge research expertise. The well-known “stable isotope probing” (SIP) of DNA and RNA has been pioneered by my group for many years and we continue to push the development of such technologies to grasp the functionalities of key microbial populations in water, soil, the rhizosphere, food webs and microbe-host systems. Recently, we have combined SIP with targeted environmental transcriptomics, which allows, for the first time, process-relevant gene expression within natural microbial communities. We have a long-standing practice of availing this expertise to project partners in national and international research collaborations.
To a large part, our research is driven by the development and application of advanced molecular ‘omics’ technologies in combination with targeted labelling, where our group hosts a distinctive and cutting-edge research expertise. The well-known “stable isotope probing” (SIP) of DNA and RNA has been pioneered by my group for many years and we continue to push the development of such technologies to grasp the functionalities of key microbial populations in water, soil, the rhizosphere, food webs and microbe-host systems. Recently, we have combined SIP with targeted environmental transcriptomics, which allows, for the first time, process-relevant gene expression within natural microbial communities. We have a long-standing practice of availing this expertise to project partners in national and international research collaborations.