Background
The importance of soils as regulators for the climate and for many environmental processes in general has given rise to a lot of attention during the previous years. Nonetheless, soils, due to their multi-disciplinary and opaque nature, are still unknown systems with regard to the many different physical, chemical and biological processes that take place simultaneously, their interactions and their scale dependency. Even overstudied processes like water flow in soils, or root water uptake are still not well understood and our theoretical tools fail to describe observations.Research Philosophy
Our research aims at the understanding and characterisation of mass and energy fluxes in soils. In that context, we are exploring from the soil physics perspective, the non-linear feedbacks between plants, soil ecology, soil chemistry and soil hydrology. Our scientific curiosity is focused on the understanding and the analysis between the discrepancy of theoretical predictions and actual observations (direct or indirect) in soils. Key element in our research is the use/development of mathematical models as representational vehicles or media. In this perspective, we are considering models as attempts to represent dependence relationships that might exist between parts of the real system. Our research focused on topics where the existing theory fails to describe new observations or when there are systematic discrepancies which cannot be explained by the current theory/models.We are working towards developing new theory and test it against the new data. This is very important in soil biophysics and generally in environmental sciences, since due to the last years’ technological advances, new types of observations are quickly made available in highly temporal and spatial resolutions, and established theories often fail to describe them. We are also interested in exploring what characterises a scientific theory as good (or bad) or in a more applied context, what data accuracy and which data-model integration characteristics/methods are required for a model output to be considered reliable.
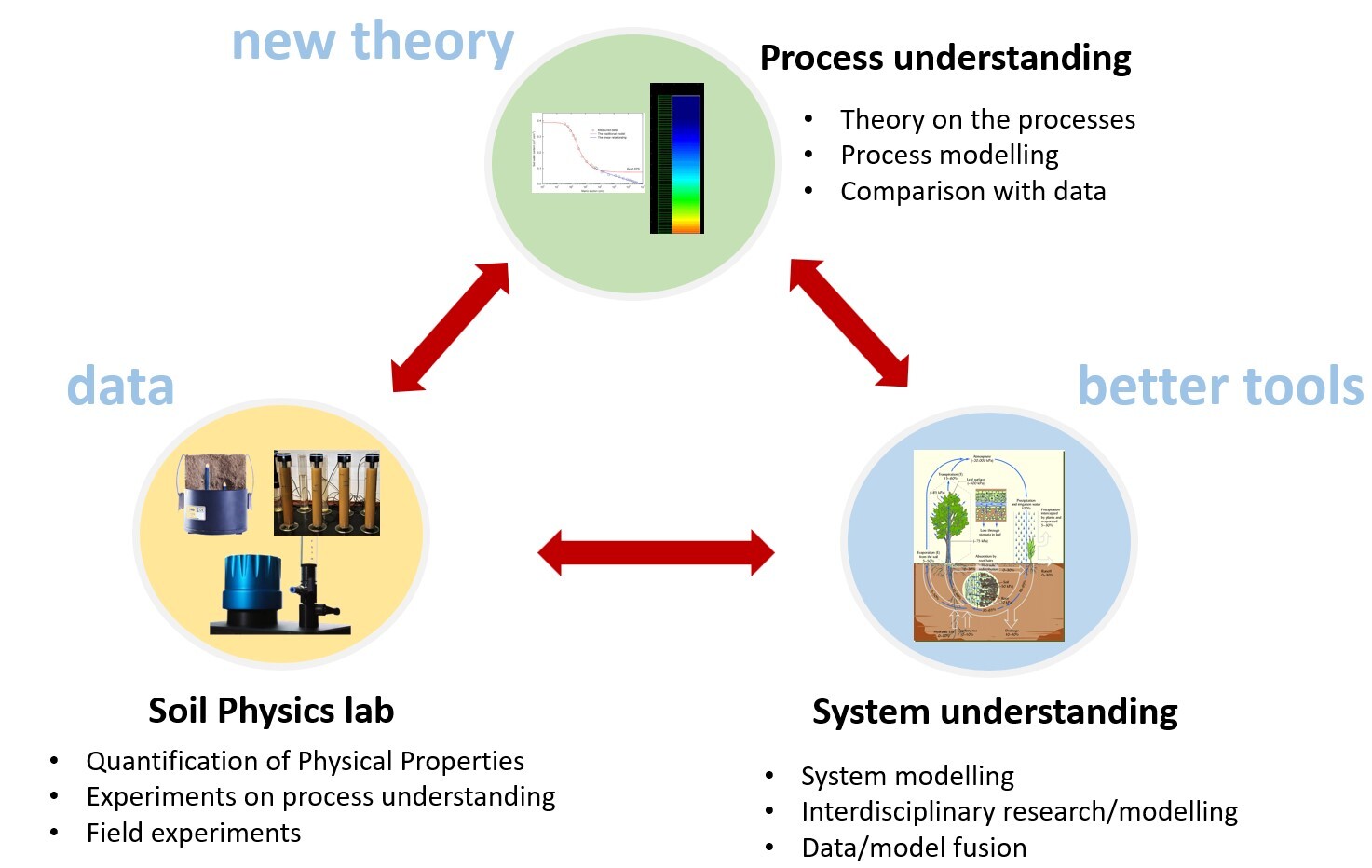
Figure 1: Research activities in the soil physics group
Methods
To achieve these objectives, we combine (Fig. 1):
- Experimental quantification of water flow, solute transport, and heat flow in soils at various spatial and temporal scales,
- Characterizing soil physical properties at the pore and at the continuum scale,
- Advanced numerical models for simulating water flow, solute transport and heat flow in soils including non-equilibrium phenomena (e.g., preferential flow),
- Multidisciplinary modeling tools describing the soil-plant-atmosphere continuum (Daisy, daisy.ku.dk)
- Inverse modelling methods, including the quantification of parameter and model prediction uncertainty

